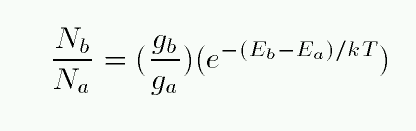The number of photons of light having wave number x in 1 J of energy source is (Plancks constant = h, velocity of light = c):
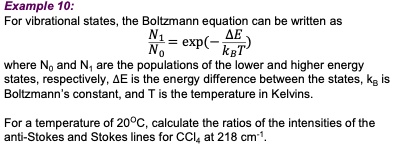
SOLVED: Example 10: For vibrational states, the Boltzmann equation can be written as exp( where No and N, the populations of the lower and higher energy states, respectively, 4E is the energy
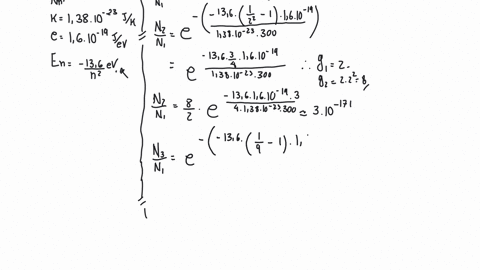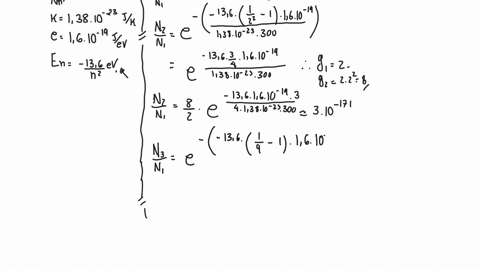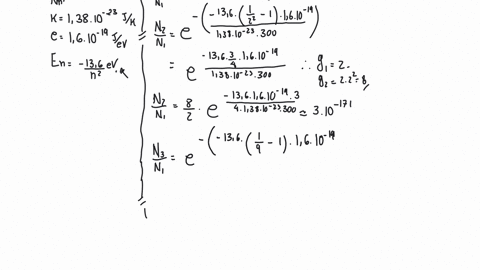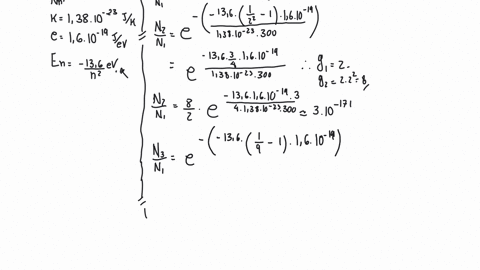
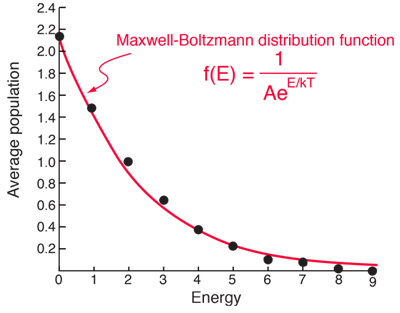
SOLVED:The energy difference between the first excited state of mercury and the ground state is 4.86 eV. (a) If a sample of mercury vaporized in a flame contains 10^20 atoms in thermal

Quantum Chemical Calculation and Evaluation of Partition Coefficients for Classical and Emerging Environmentally Relevant Organic Compounds | Environmental Science & Technology
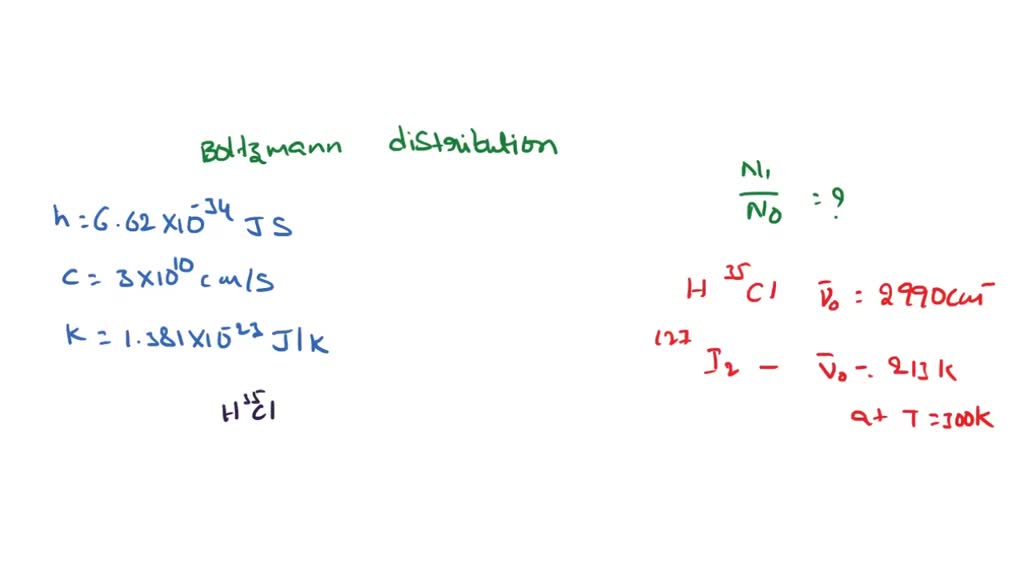
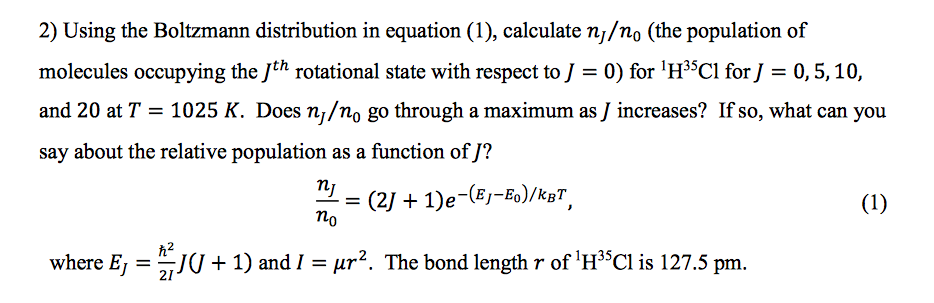
SOLVED: The wavenumber of the fundamental transition of 'HIP9F is 3958 cm !, Calculate the relative population between the first and ground vibrational states of 'H"PF at temperature of 298 How would

If levels 1 and 2 are separated by an energy E2 - E1 , such that the corresponding transition frequency falls in the middle of the visible range, calculate the ratio of