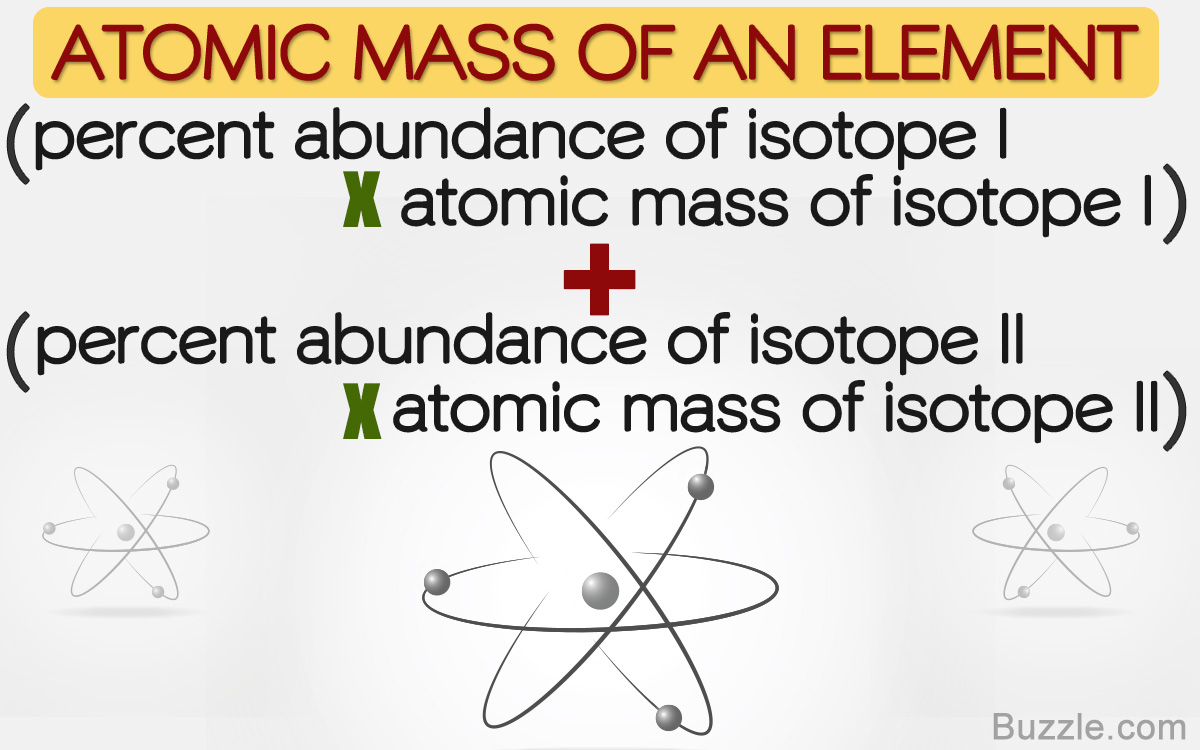
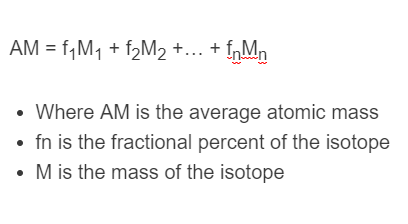
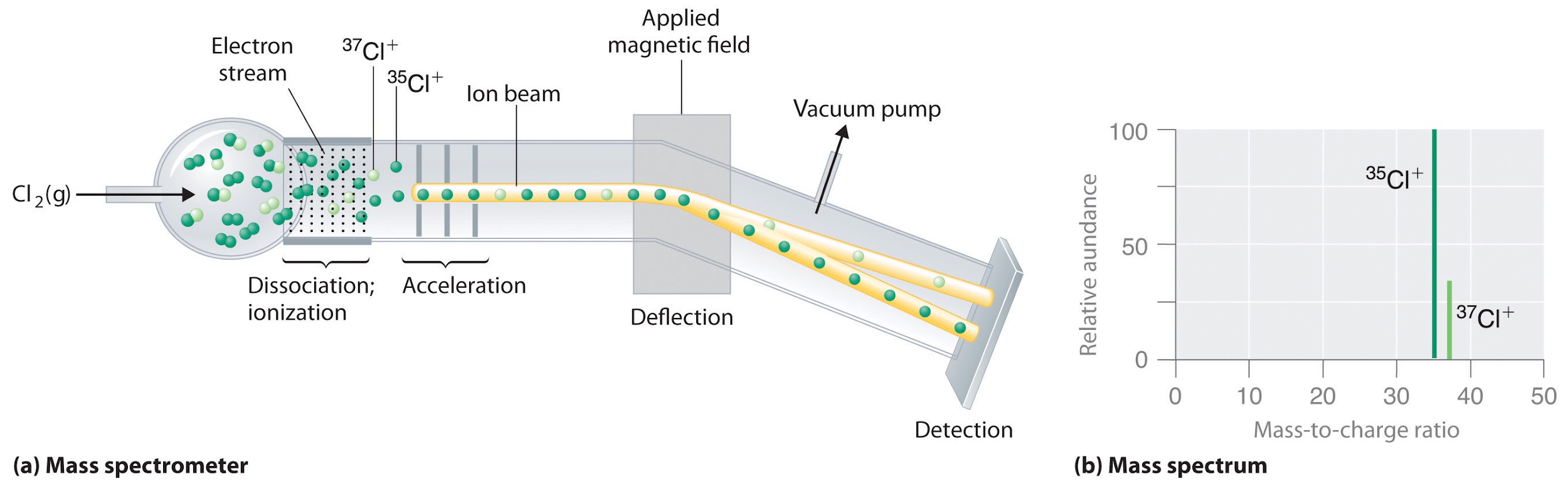
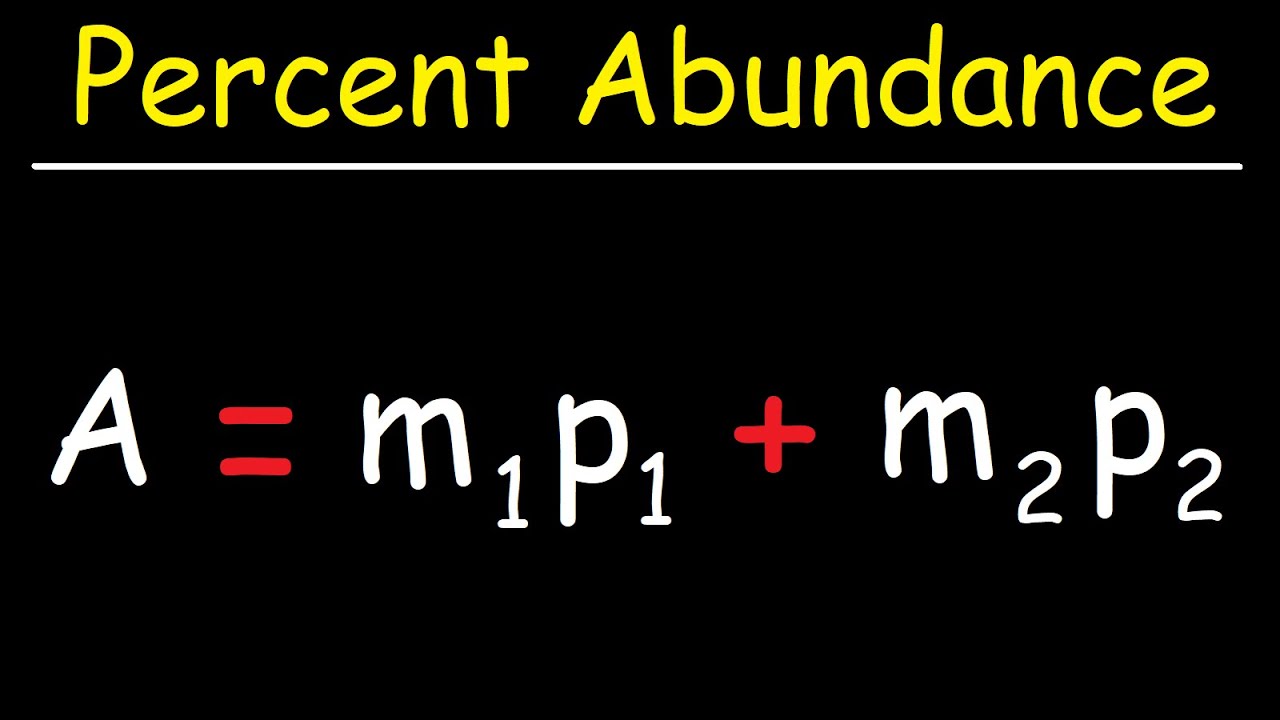
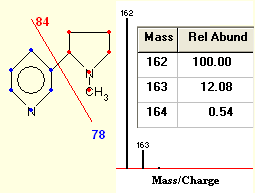
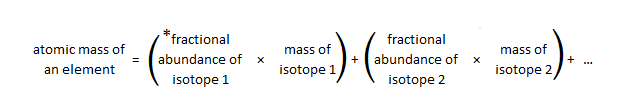How would you calculate the relative abundance for two isotopes when the relative atomic mass is given? - Quora

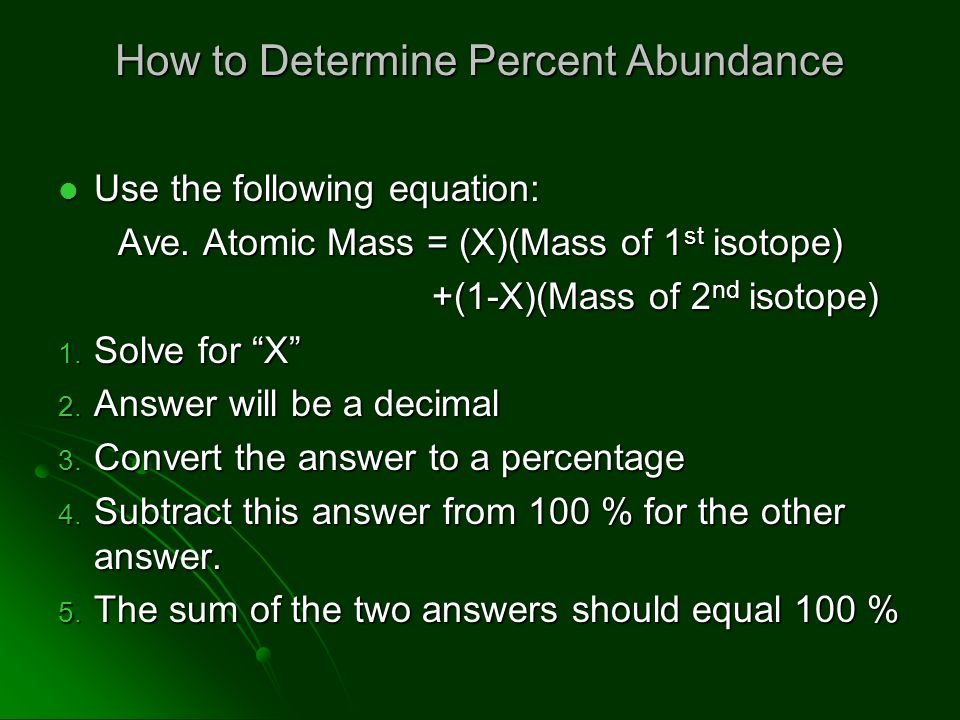
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their

How to Solve for Percent Abundance of Isotopes Examples, Practice Problems, Step by Step Explanation - YouTube