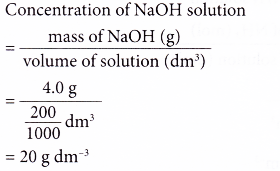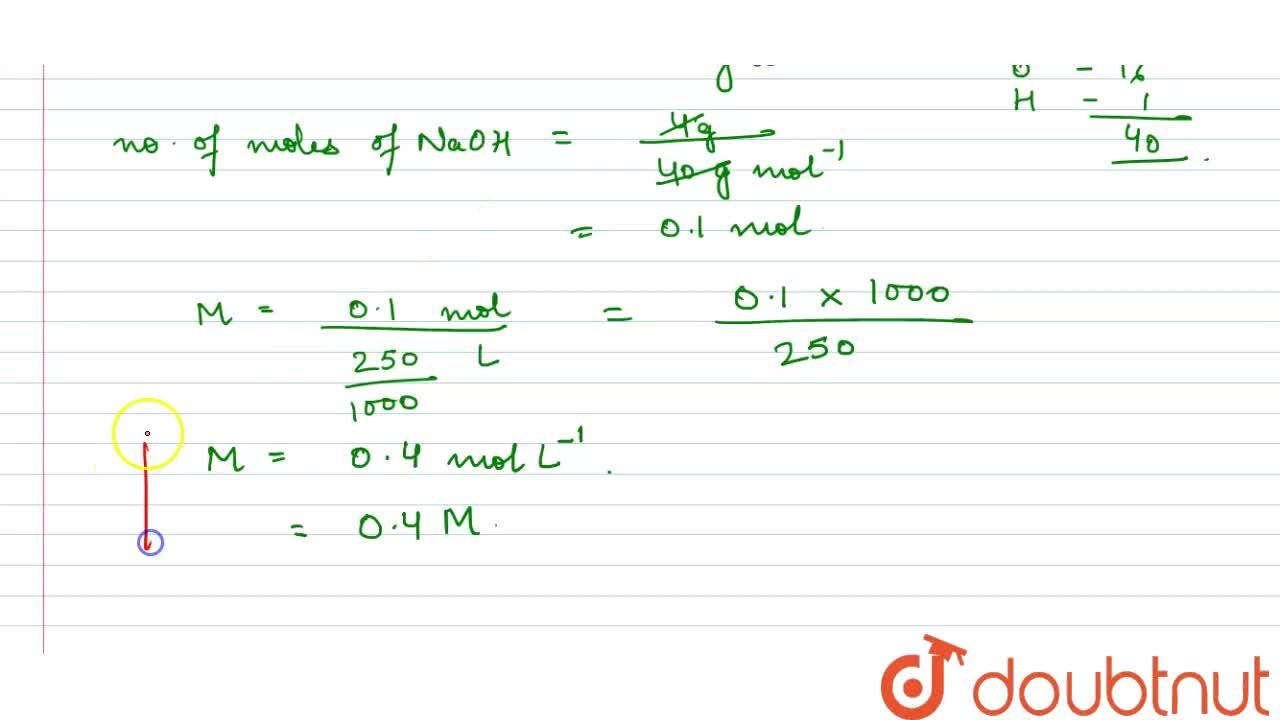
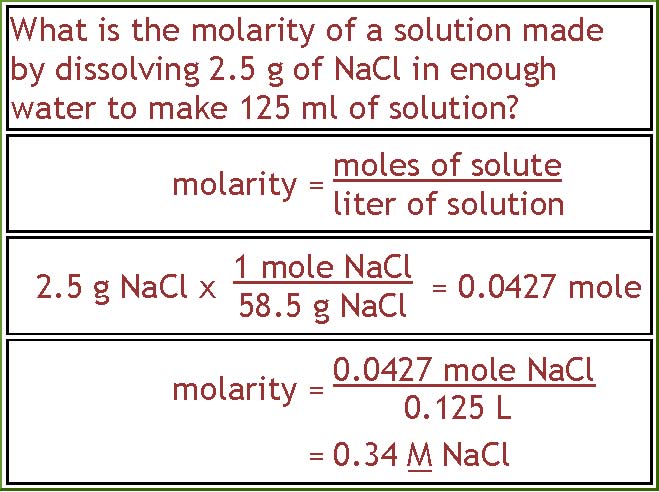
Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to form 250 mL of the solution.
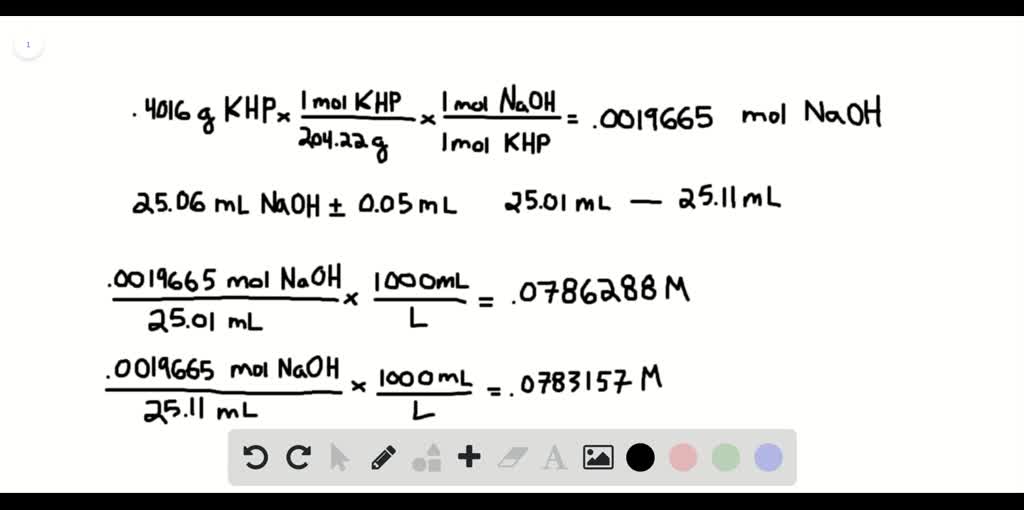
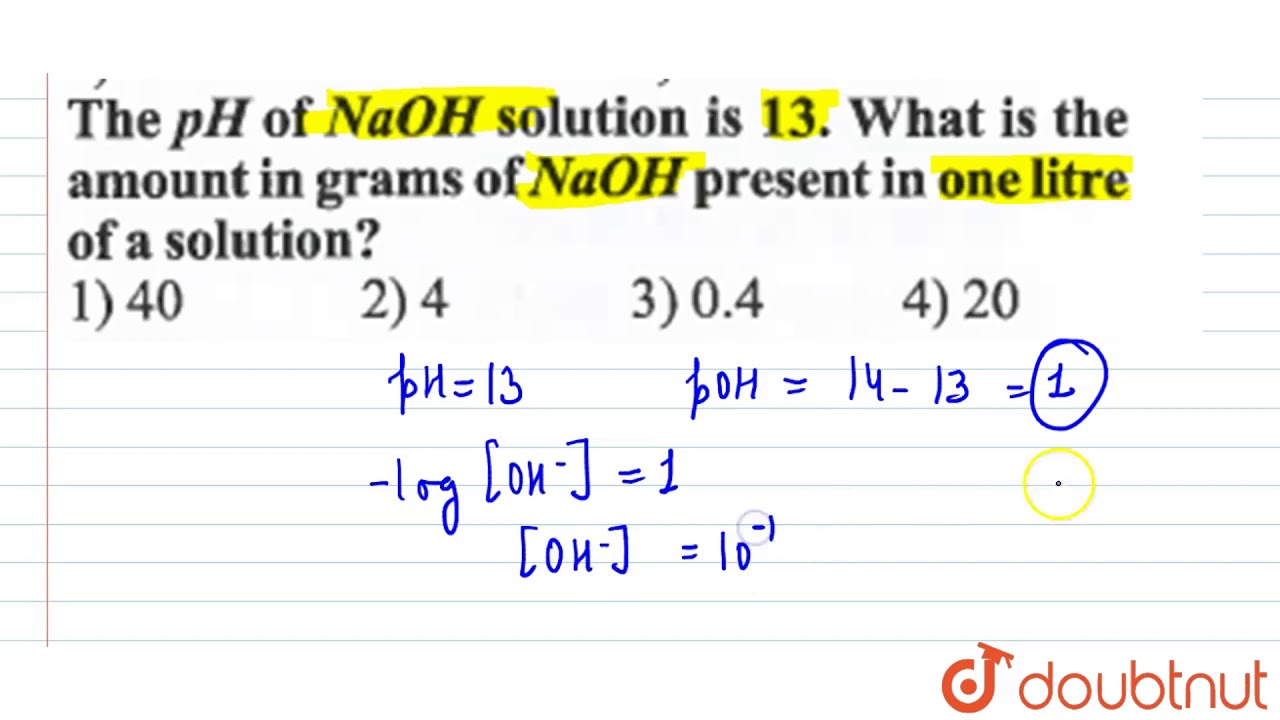
SOLVED:It took 25.06 ±0.05 mL of a sodium hydroxide solution to titrate a 0.4016-g sample of KHP (see Exercise 79). Calculate the concentration and uncertainty in the concentration of the sodium hydroxide
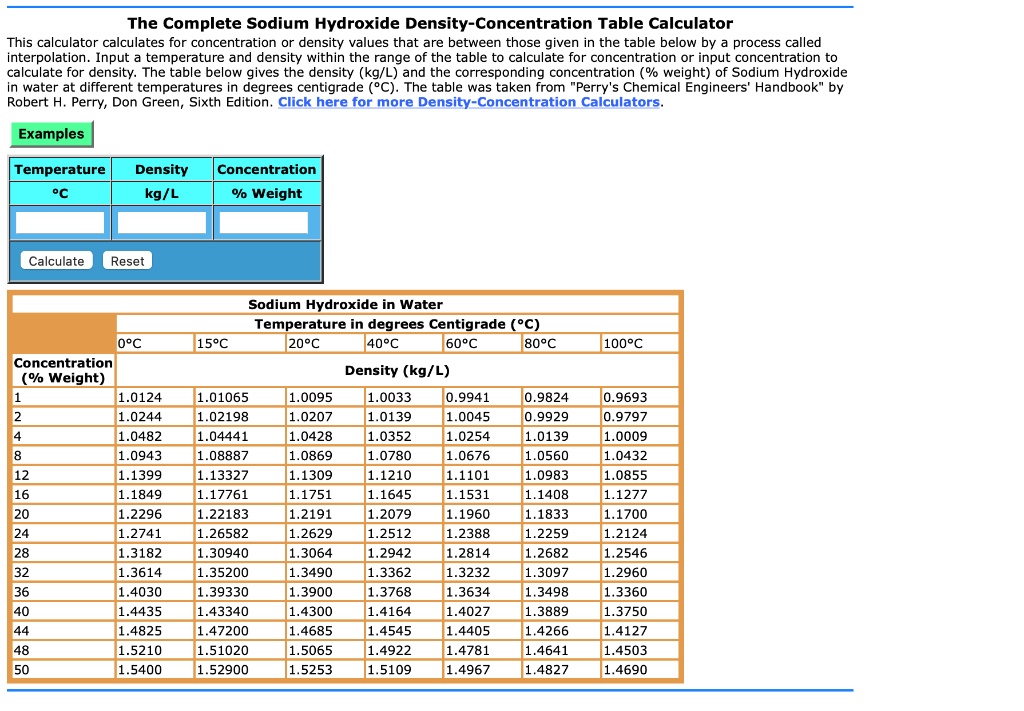
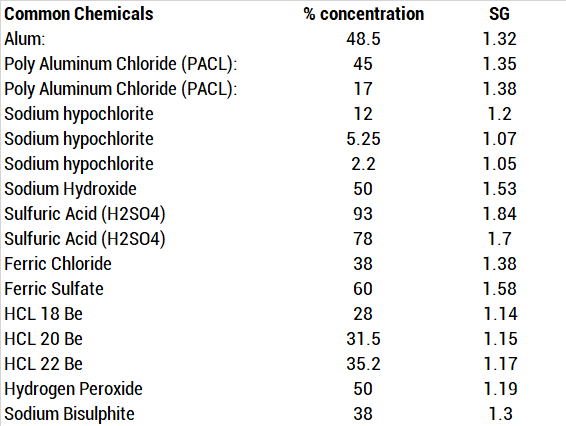
SOLVED: The Complete Sodium Hydroxide Density-Concentration Table Calculator This calculator calculates for concentration or density values that are between those given in the table below by process called interpolation. Input temperature and

Molarity of NaOH in a solution prepared by dissolving 4 g of NaOH in enough water to form 250 ml of solution is:A. 0.4 MB. 4.1 MC. 1.4 MD. 0.45 M

Calculate the molarity of NaOH in the solution prepared by dissolving its 4 g in enough water to... - YouTube

How to Calculate Molarity- With Tricks मोलरिटी आसान ट्रिक्स GPAT-NIPER-Pharmacist Exam | Model question paper, Question paper, Online tests
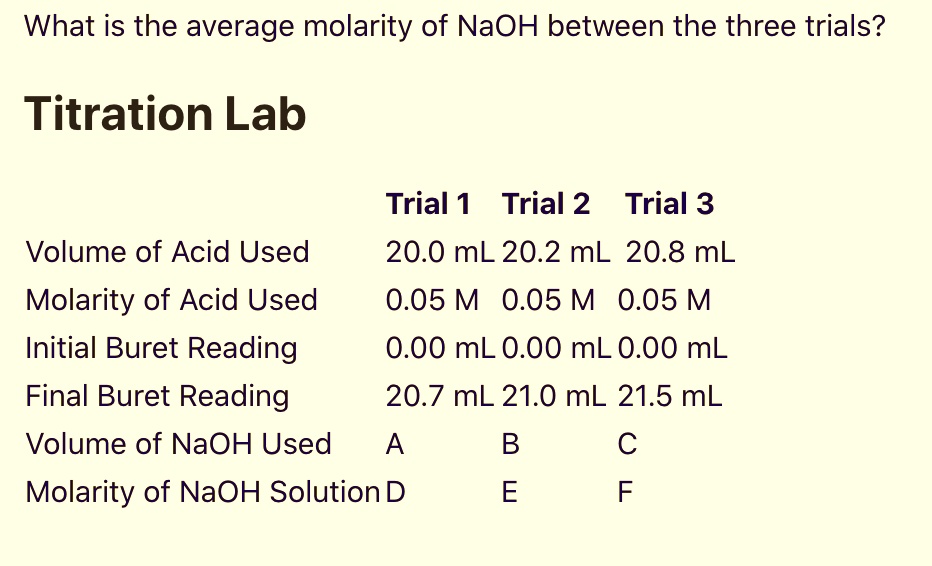
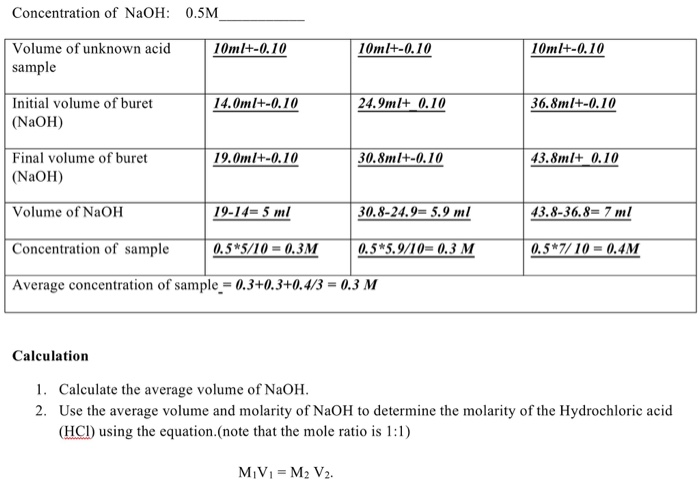
SOLVED: What is the average molarity of NaOH between the three trials? Titration Lab Trial 1 Trial 2 Trial 3 20.0 mL 20.2 mL 20.8 mL 0.05 M 0.05 M 0.05 M


![Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube Calculating pH from [OH-] hydroxide Concentration - CLEAR & SIMPLE - YouTube](https://i.ytimg.com/vi/gn1CgBzShps/maxresdefault.jpg)
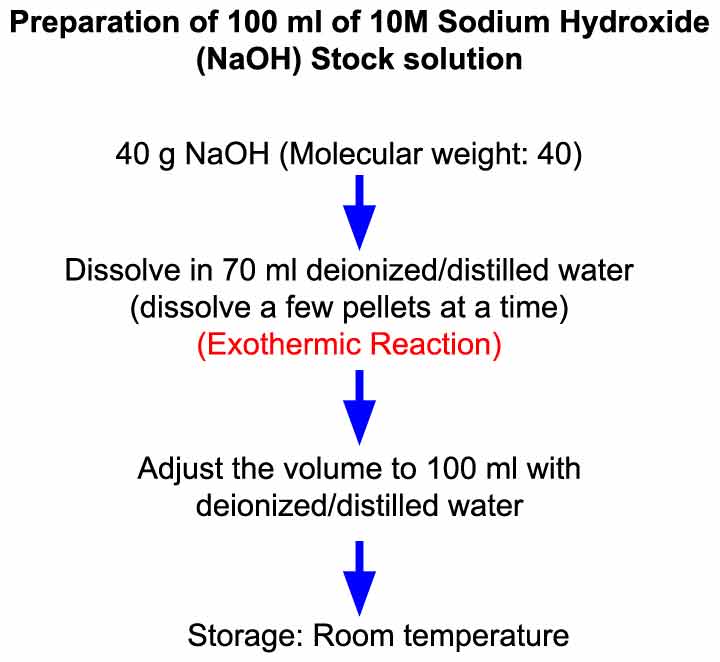
:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)