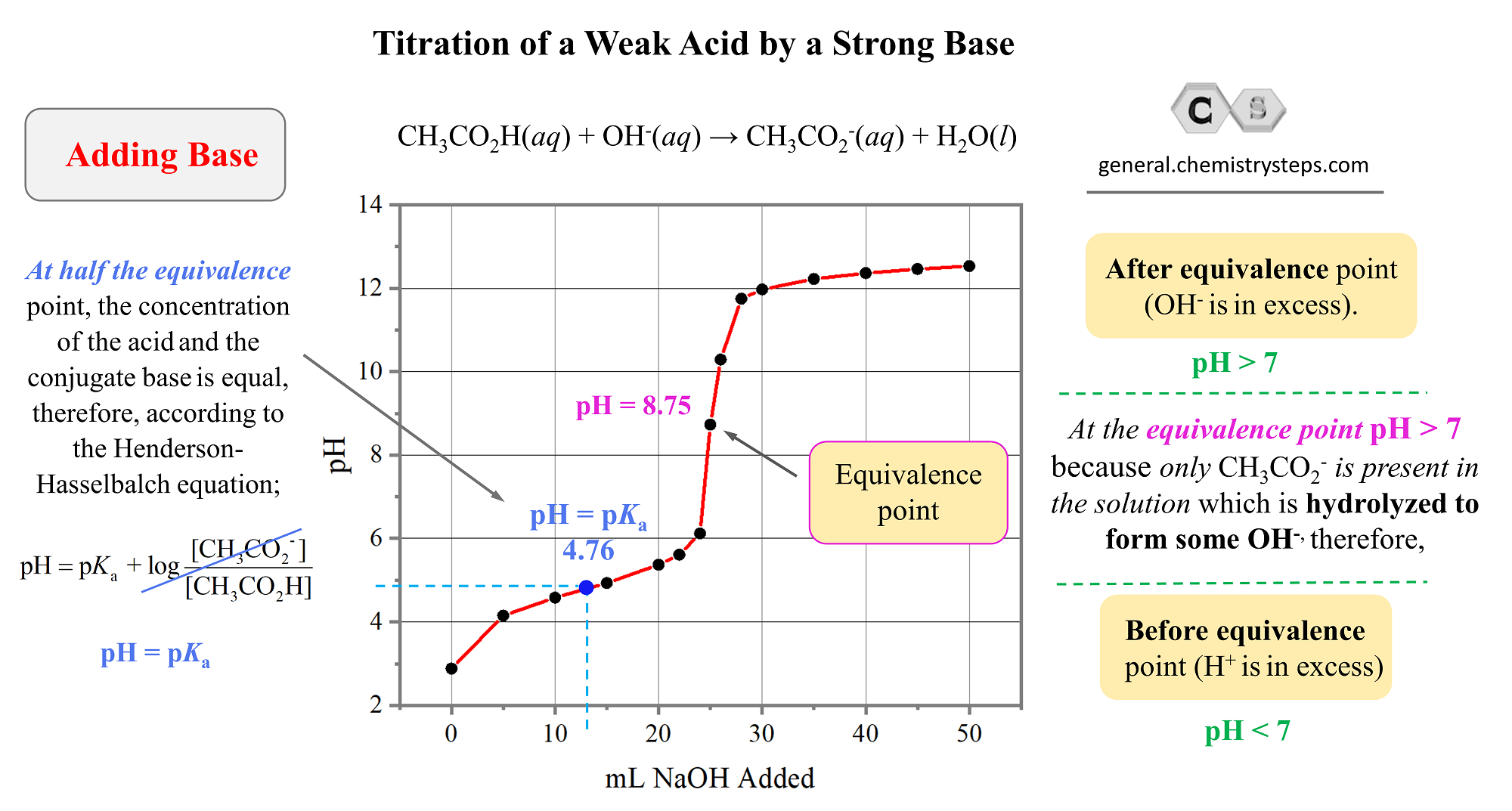
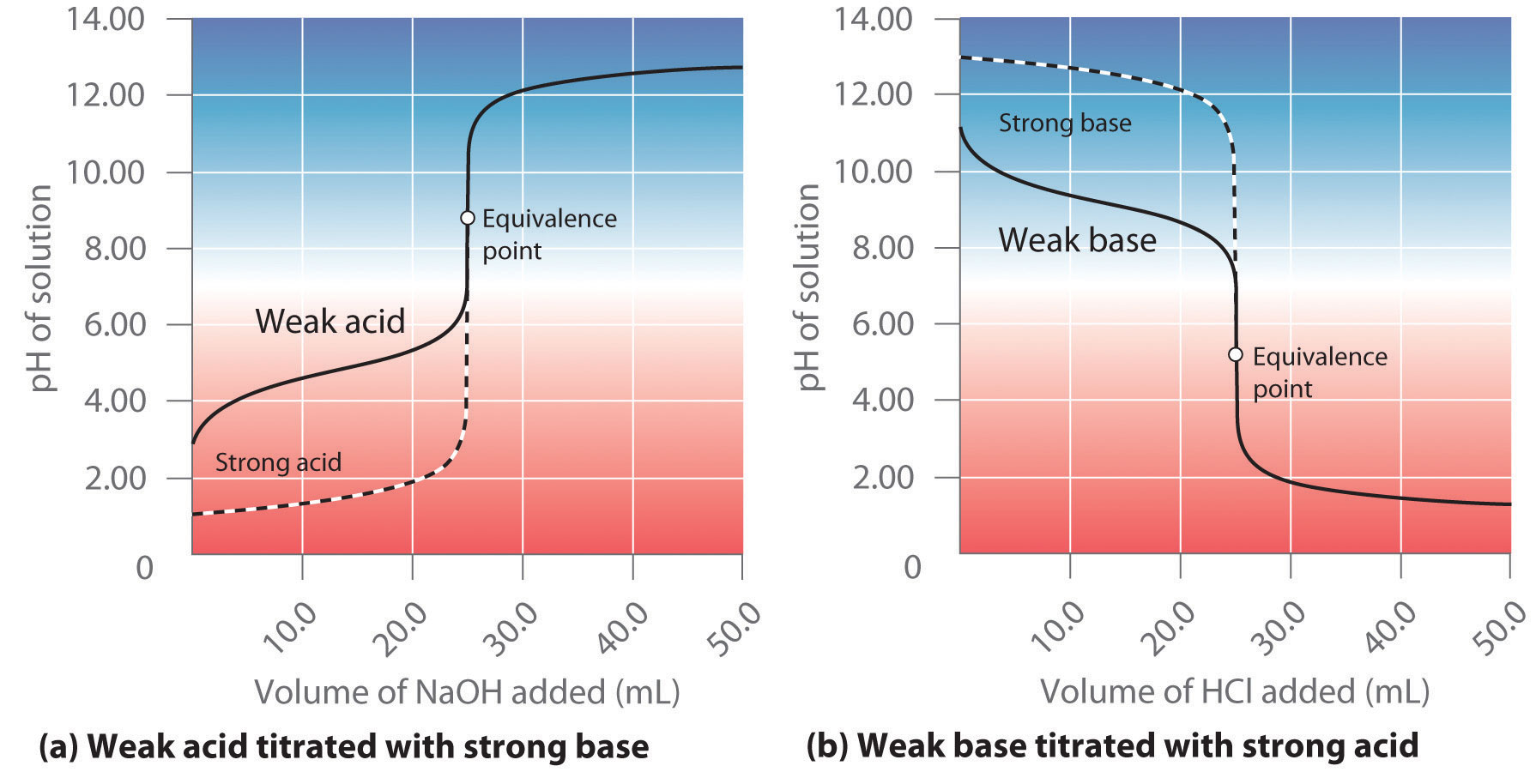
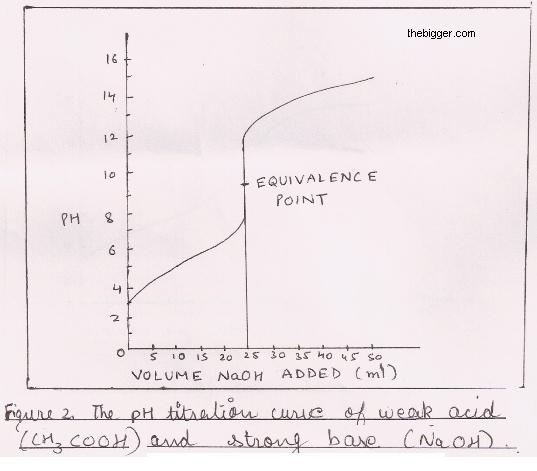
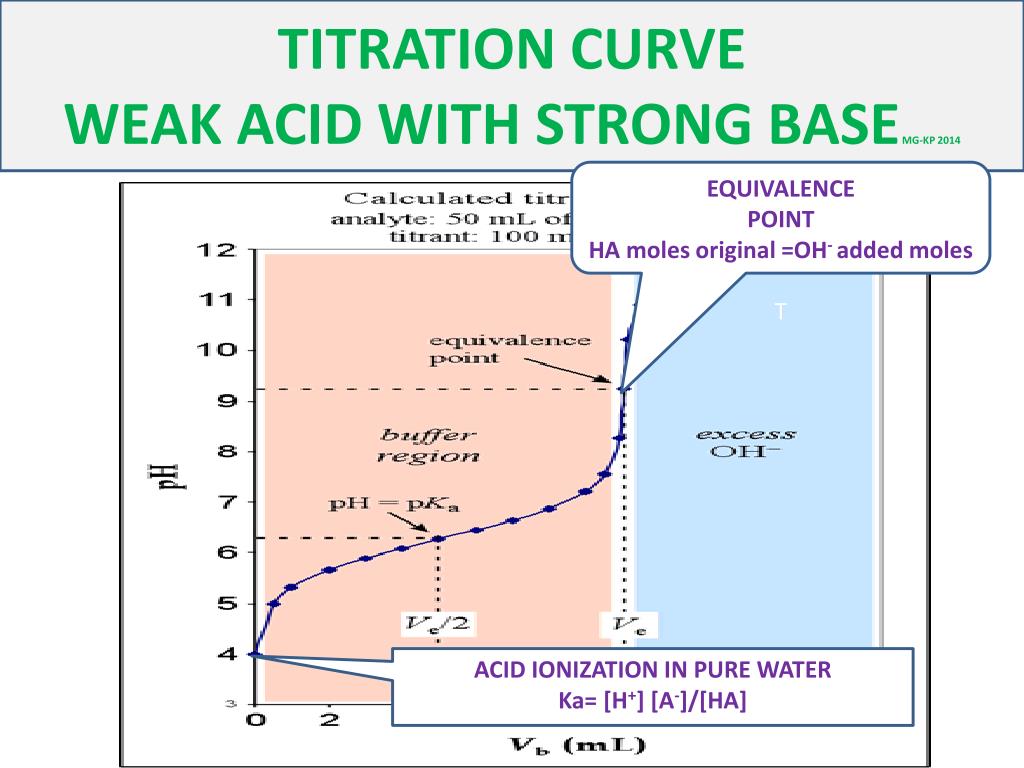
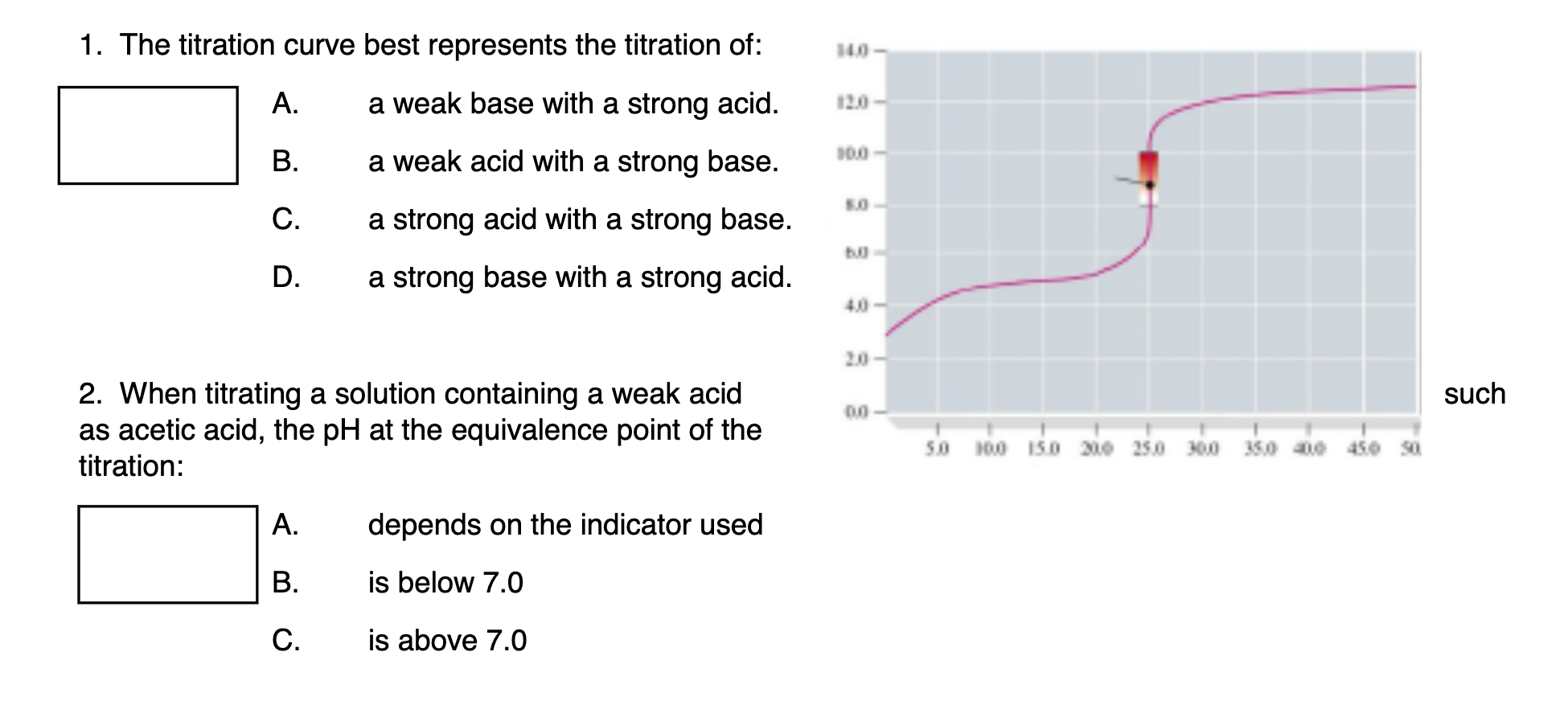
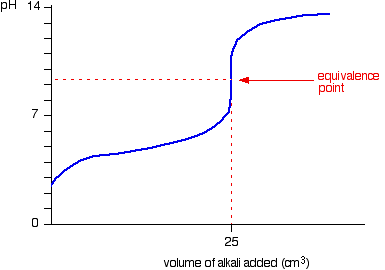
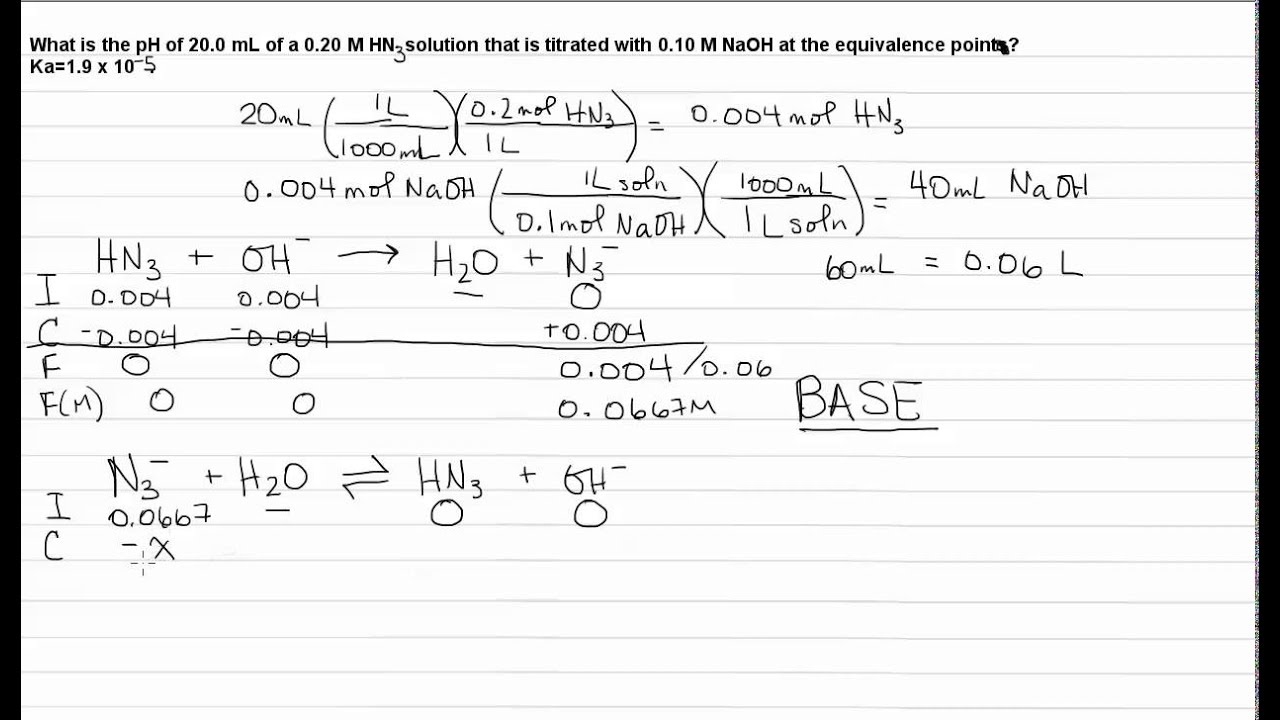
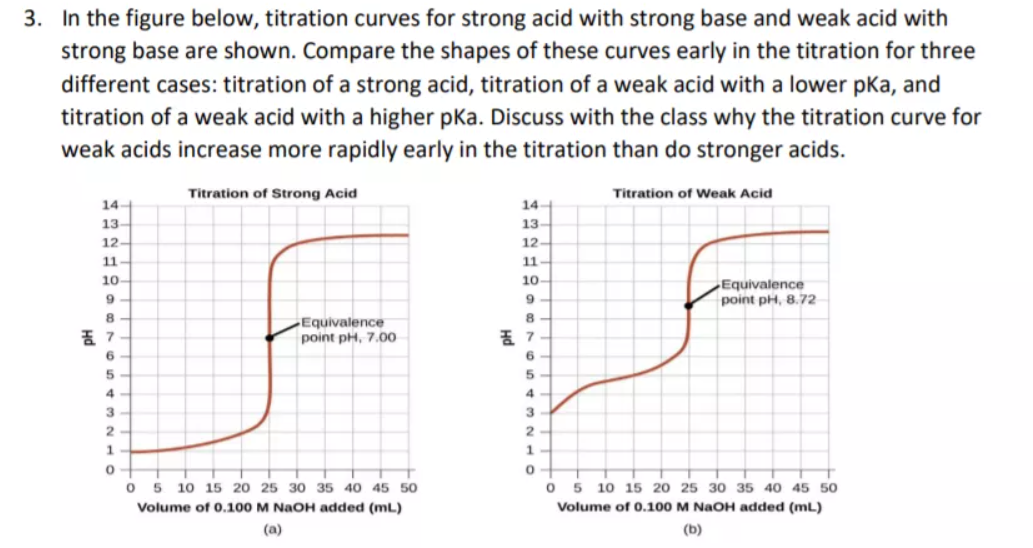
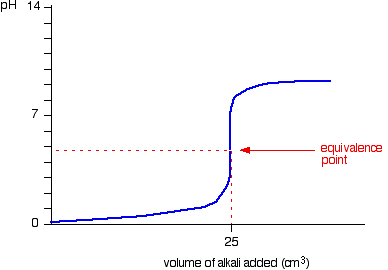
Which of the following pH curve represent the titration of weak acid and strong base (dotted line show equivalence point)?

Sketch the following titration curves. a) A strong acid/strong base. b) A weak monoprotic acid/strong base. c) A weak diprotic acid/strong base. | Homework.Study.com