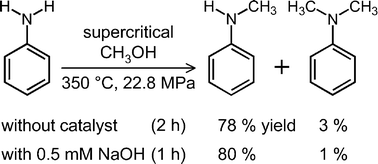
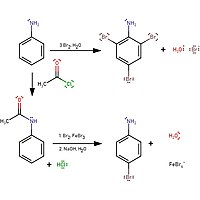
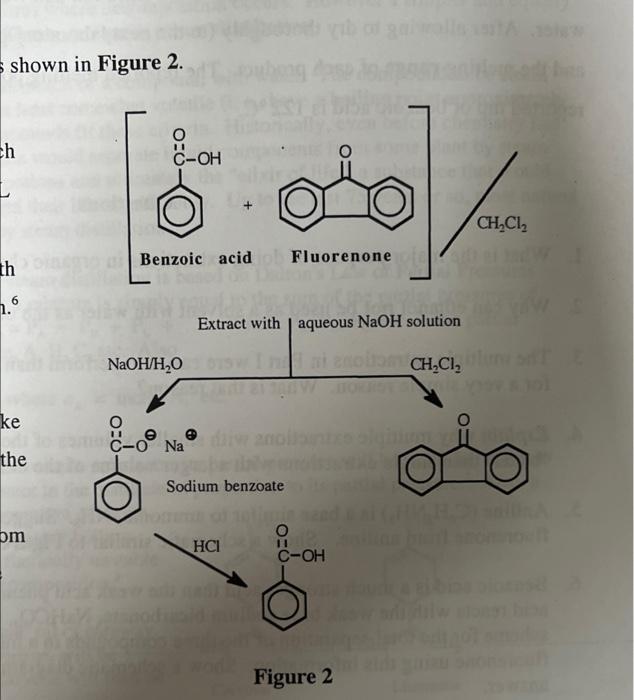
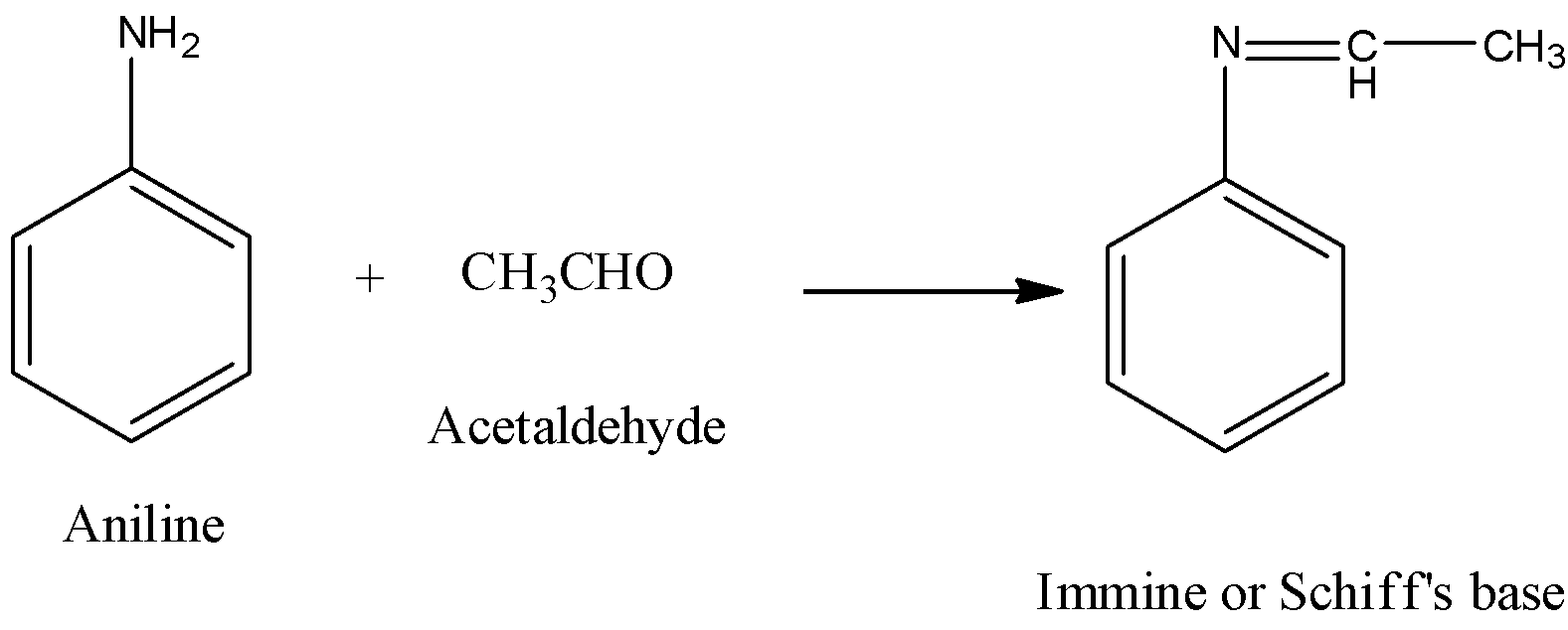
Propose a method to separate the following three compounds: benzoic acid, aniline and naphthalene. How would this separation look in the form of a schematic diagram? | Homework.Study.com

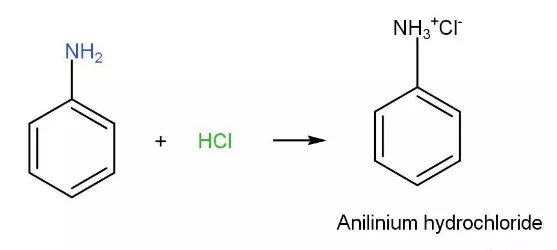
OneClass: Aniline (17), an amine, is soluble in diethyl ether but not water; however, aniline is extr...
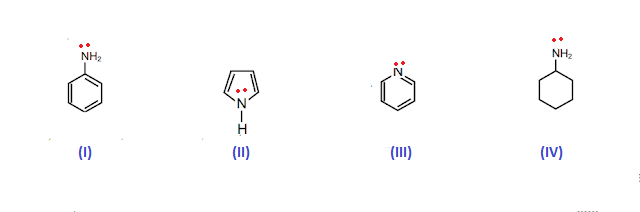
Welcome to Chem Zipper.com......: Arrange in correct order of basic Character of aniline, pyrrol, pyridine and piperidine?
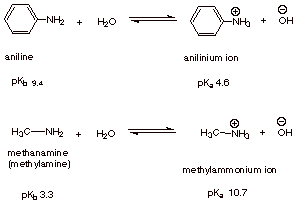
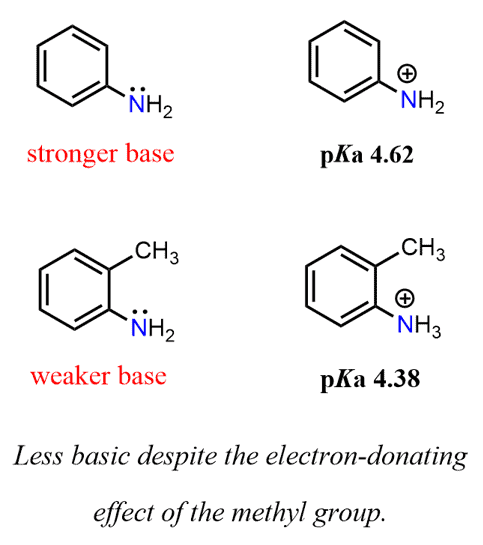
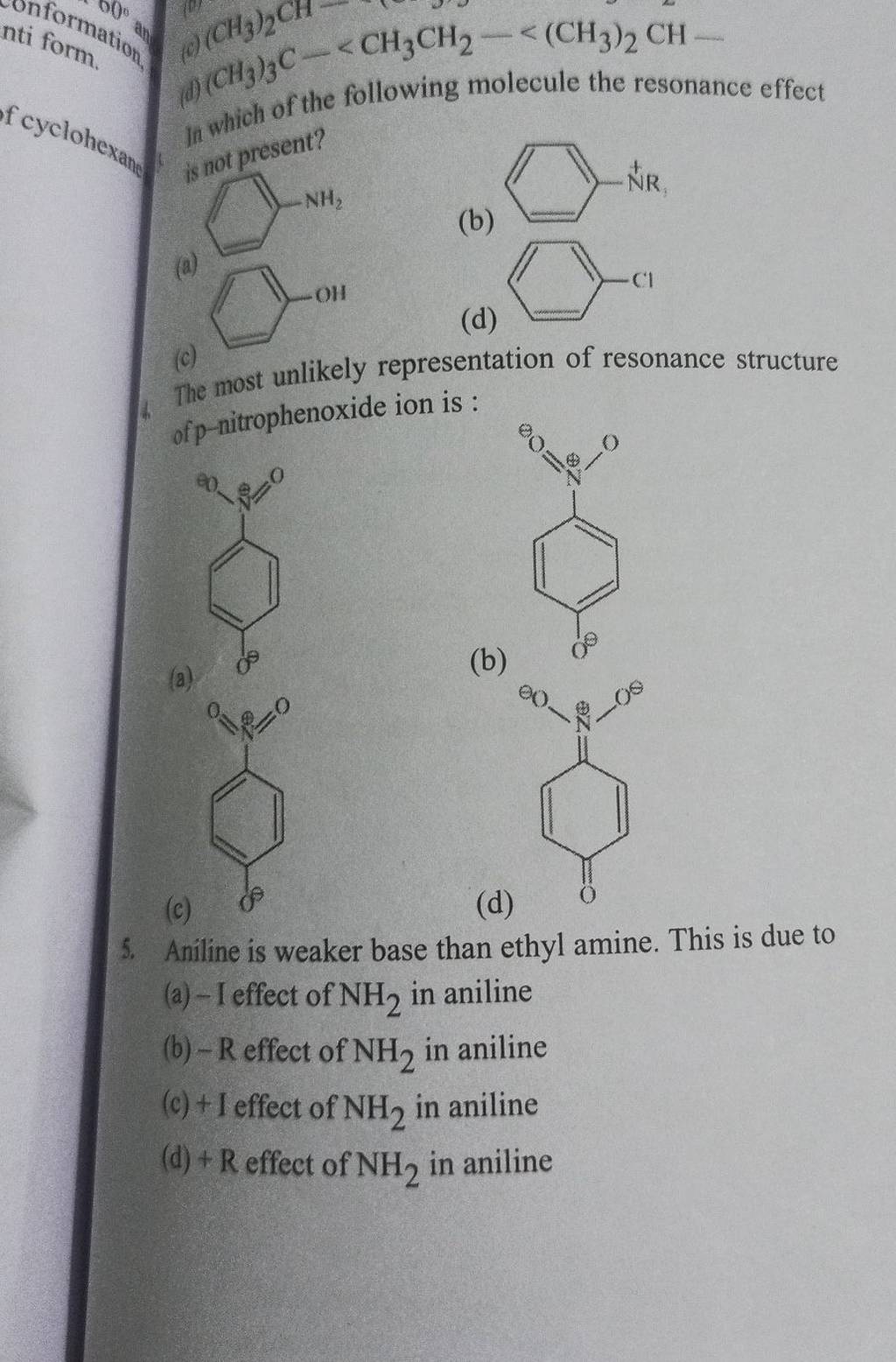
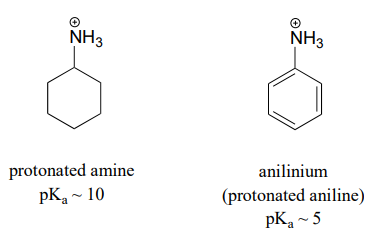
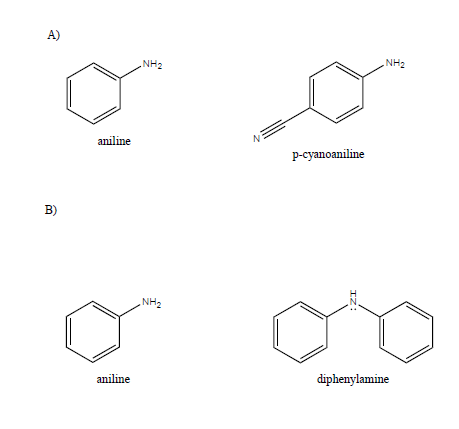
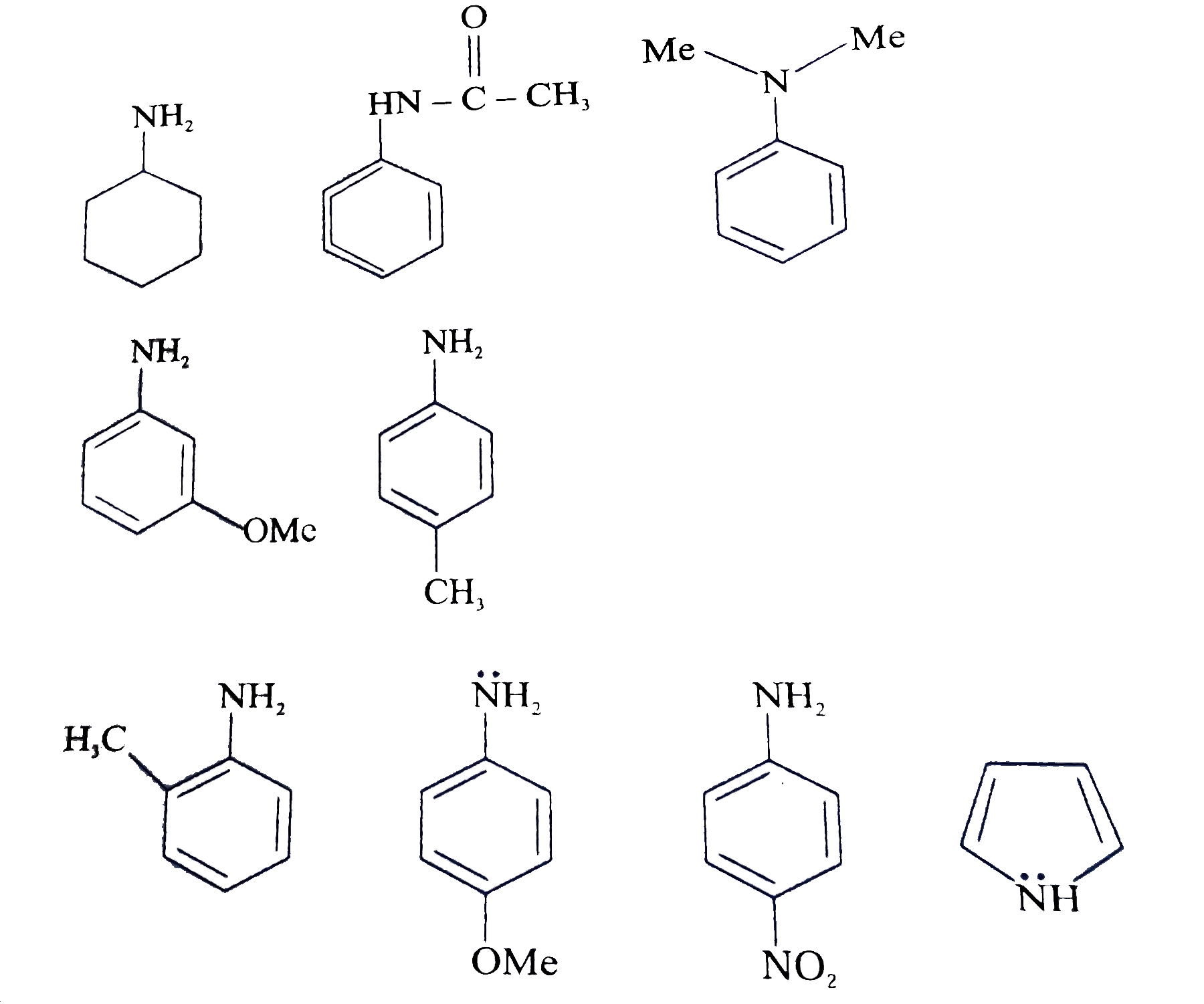
Arrange the following as directed: (a) Increasing order of basic strength: Aniline, p – nitroaniline and p – toluidine. (b) Decreasing order of basic strength in gas phase: C2H5NH2, (C2H5)2NH, (C2H5)3N and

Aniline monomer, its polymerized forms and possible state of oxidized... | Download Scientific Diagram